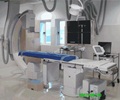
Abbott’s drug eluting stents, XIENCE PRIME™ Everolimus Eluting Coronary Stent System and the XIENCE V® Everolimus Eluting Coronary Stent System have both received additional new CE Markings (Conformité Européenne), covering the treatment of patients with diabetes.
The CE marking certifies that a product has met EU consumer safety, health or environmental requirements.Coronary artery disease is one of the most common cardiovascular complications of diabetes and is the number one cause of death among European adults with diabetes.
The high blood glucose levels associated with diabetes can lead to increased deposits of fatty materials on the insides of the blood vessel walls. These deposits may affect blood flow to the heart, increasing the chance of clogging and hardening of blood vessels.
Patients with diabetes who also have coronary artery disease often have poorer outcomes after stent procedures because their blood vessels tend to build up more plaque than the vessels of non-diabetic patients, and their coronary artery disease advances more quickly.
"This expanded indication further confirms XIENCE PRIME and XIENCE V as important options for physicians who are treating patients with diabetes," said Charles Simonton, M.D., FACC, FSCAI, divisional vice president, Medical Affairs, and chief medical officer, Abbott Vascular. "Patients with diabetes tend to be sicker and have more challenging anatomy, such as small vessels or long lesions, which can be difficult to treat.
The deliverability of both devices provides physicians with confidence to easily reach the lesion site."
Advertisement
The expanded indications for XIENCE PRIME and XIENCE V are based on randomized clinical trial data from the SPIRIT family of trials that support the safety and performance of the stents in these patient subgroups. XIENCE PRIME also is indicated for use in long vessels and is available in stent lengths of 33 mm and 38 mm. Both XIENCE PRIME and XIENCE V leverage the excellent outcomes from the extensive body of clinical evidence from the SPIRIT family of clinical trials.
Advertisement
XIENCE PRIME received CE Mark in June 2009, and XIENCE V received CE Mark in 2006. XIENCE PRIME is an investigational device in the United States and is not available for sale in the United States or outside of jurisdictions in which the product has been CE Marked. These additional indications apply to XIENCE V exclusively in countries where the product bears a CE Mark.
Source-Medindia
GPL